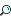The Periodic Table and Periodic Properties
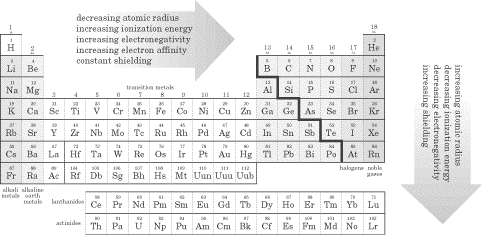
We just saw how the periodic table can help us quickly determine electron configurations and quantum numbers. As you’ll see in this section, this is possible because of the special arrangement of elements in the periodic table. There will almost definitely be at least one question about trends in the periodic table on the SAT Chemistry test, so be sure to read this section closely. The Anatomy of the Periodic Table As you are probably well aware, in the periodic table, elements are arranged in order of increasing atomic number. The 18 vertical columns of the table are called groups or families, while the seven horizontal rows are called periods and correspond to the seven principal quantum energy levels, n = 1 through n = 7.
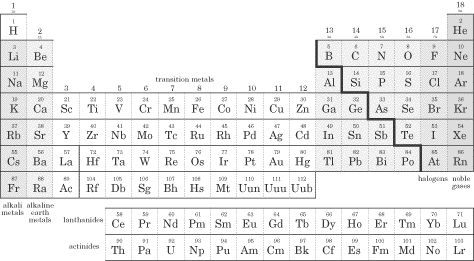
On the right side of the periodic table is a dividing line resembling a staircase. To the left of the staircase lie the metals, and to the right of the staircase lie the nonmetals. Many of the elements that touch the staircase are called metalloids, and these exhibit both metallic and nonmetallic properties. Study the diagram below and memorize the names of the different types of elements, because you will definitely see questions about these groupings on the test!
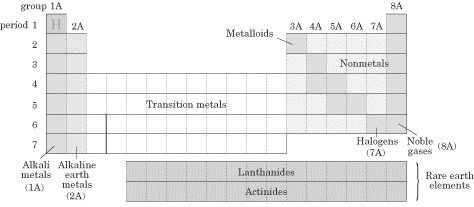
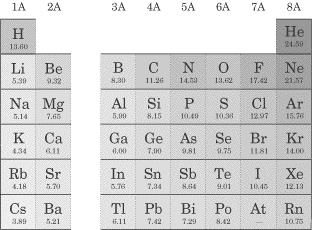
Metals are malleable, ductile, and have luster; most of the elements on the periodic table are metals. They oxidize (rust and tarnish) readily and form positive ions (cations). They are excellent conductors of both heat and electricity. The metals can be broken down into several groups. Transition metals (also called the transition elements) are known for their ability to refract light as a result of their unpaired electrons. They also have several possible oxidation states. Ionic solutions of these metals are usually colored, so these metals are often used in pigments. The actinides and lanthanides are collectively called the rare earth elements and are filling the f orbitals. They are rarely found in nature. Uranium is the last naturally occurring element; the rest are man-made. Nonmetals lie to the right of the staircase and do not conduct electricity well because they do not have free electrons. All the elemental gases are included in the nonmetals. Notice that hydrogen is placed with the metals because it has only one valence electron, but it is a nonmetal. Here are some specific families you should know about, within the three main groups (metals, nonmetals, and metalloids): Alkali metals(1A)—The most reactive metal family, these must be stored under oil because they react violently with water! They dissolve and create an alkaline, or basic, solution, hence their name. Alkaline earth metals (2A)—These also are reactive metals, but they don’t explode in water; pastes of these are used in batteries. Halogens (7A)—Known as the “salt formers,” they are used in modern lighting and always exist as diatomic molecules in their elemental form. Noble gases (8A)—Known for their extremely slow reactivity, these were once thought to never react; neon, one of the noble gases, is used to make bright signs. Now that you’re familiar with the different groupings of the periodic table, it’s time to talk about the ways we can use the periodic table to predict certain characteristics of elements. Atomic Radius Since in an atom there is no clear boundary beyond which the electron never strays, the way atomic radius is measured is by calculating the distance between the two nuclei of atoms when they are involved in a chemical bond. If the two bonded atoms are of the same element, you can divide the distance by 2 to get the atom’s radius. That said, one of the two important things you’ll need to know about atomic radii for the SAT II Chemistry exam is that atomic radii decrease moving across a period from left to right. But why? It seems as though the more protons you add, the more space the atom should take up, but this is not the case. The reason for this lies in the basic concept that opposite charges attract each other and like charges repel each other. As you increase the number of protons in the nucleus of the atom, you increase the effective nuclear charge of the atom (Zeff), and the nucleus pulls more strongly on the entire electron cloud. This makes the atomic radius decrease in size. The second thing you’ll need to know is that atomic radii increase moving down a group or family. This is easier to understand if you refer to the Bohr model. As you move down the table, the value of n increases as we add another shell. Remember that the principal quantum number, n, determines the size of the atom. As we move down a family, the attractive force of the nucleus dissipates as the electrons spend more time farther from the nucleus. One more thing about atomic size. As you know, when an atom loses an electron, a cation, or positive ion, is formed. When we compare the neutral atomic radius to the cationic radius, we see that the cationic radius is smaller. Why? The protons in the nucleus hold the remaining electrons more strongly. As you might expect, for negatively charged ions, or anions, the nuclear attractive force decreases (and there is enhanced electron-electron repulsion), so the electrons are less tightly held by the nucleus. The result is that the anion has a larger radius than the neutral atom.The SAT II Chemistry test might ask you to compare the sizes of two atoms that are isoelectronic, meaning that they have the same number of electrons. In this case, you would then consider the number of protons the two atoms possess. Example Which ion is larger, F– or O2-? Explanation Since these two atoms are isoelectronic and in the same period, the atom with more protons in its nucleus will hold its electrons more tightly and be smaller. Fluoride will be smaller since it has more protons (9, compared to oxide’s 8). Ionization Energy (IE) The ionization energy of an atom is the energy required to remove an electron from the atom in the gas phase. Although removing the first electron from an atom requires energy, the removal of each subsequent electron requires even more energy. This means that the second IE is usually greater than the first, the third IE is greater than the second, and so on. The reason it becomes more difficult to remove additional electrons is that they’re closer to the nucleus and thus held more strongly by the positive charge of the protons. Ionization energies differ significantly, depending on the shell from which the electron is taken. For instance, it takes less energy to remove a p electron than an s electron, even less energy to extract a d electron, and the least energy to extract an f electron. As you can probably guess, this is because s electrons are held closer to the nucleus, while f electrons are far from the nucleus and less tightly held. You’ll need to remember two important facts about ionization energy for the test. The first is that ionization energy increases as we move across a period. The reason for this, as is the case with periodic trends in atomic radii, is that as the nucleus becomes more positive, the effective nuclear charge increases its pull on the electrons and it becomes more difficult to remove an electron. The second thing you’ll need to remember is that ionization energy decreases as you move down a group or family. The increased distance between electrons and the nucleus and increased shielding by a full principal energy level means that it requires less energy to remove an electron. Shieldingoccurs when the inner electrons in an atom shield the outer electrons from the full charge of the nucleus. Keep in mind that this phenomenon is only important as you move down the periodic table! Here are the values for the first ionization energies for some elements:
There are some important exceptions to the above two ionization energy trends in the periodic table, so make sure you study these closely:When electron pairing first occurs within an orbital, electron-electron repulsions increase, so that removing an electron takes less energy (it’s easier); thus the IE drops at this time. For example, less energy is required to remove an electron from oxygen’s valence in spite of an increasing Zeff because oxygen’s p4 electron is the first to pair within the orbital. The repulsion created lowers the amount of energy required to remove either electron. There is also a drop in ionization energy from s2 to p1—also in spite of an increasingZeff. This drop is due to the fact that you are removing a p electron rather than an s electron. The p electrons are less tightly held because they do not penetrate the electron cloud toward the nucleus as well as an s electron does. Example Which of the following elements has the highest ionization energy: K, Ca, Ga, As, or Se? Explanation The answer is arsenic, or As. Since IE increases as we move across a period, you may have chosen Se. However, there is a drop in IE in spite of increasing Zeff due to the increased electron-electron repulsion in the family that contains oxygen, since they are np4. Electron Affinity An atom’s electron affinity is the amount of energy released when an electron is added to the atom in its gaseous state—when an electron is added to an atom, the atom forms a negative ion. Most often, energy is released as an electron is added to an atom, and the greater the attraction between the atom and the electron added, the more negative the atom’s electron affinity. For the SAT II Chemistry test, remember that electron affinity becomes more negative as we move across a period. This means that it’s easier to add an electron to elements, the farther to the right you travel on the periodic table. Why? Again, this is because the higher Zeff increases the nuclear attraction for the incoming electron. Important exceptions to this rule are the noble gases: He, Ne, Ar, Kr, and Xe. They have electron affinities that are positive (meaning very low), because if they were to accept another electron, that electron would have to go into a new, higher-energy subshell, and this is energetically unfavorable. Electron affinities do not change very much as you go down a group. This is because the lower electron-nucleus attraction that’s seen as we go down a group is pretty evenly counterbalanced by a simultaneous lowering in electron-electron repulsion. Remember that there is no clear trend for electron affinity as you go down a group on the periodic table—this fact could come up in a synthesis of knowledge question! Electronegativity Electronegativity is a measure of the attraction an atom has for electrons when it is involved in a chemical bond. Elements that have high ionization energy and high electron affinity will also have high electronegativity since their nuclei strongly attract electrons. Electronegativity increases from left to right as we move across a period and decreases as we move down any group or family. By now, these trends should make sense. You know that ionization energies tend to decrease with increasing atomic number in a group, although there isn’t a significant change in electron affinity, so it makes sense that atoms’ attraction for electrons in a bond would also increase as their Zeff increased. We will discuss the concept of electronegativity further in the next section, when we discuss chemical bonding. Here’s a summary of the trends we discussed in this section. Make sure to memorize them!
(责任编辑:申月月)
视频学习
我考网版权与免责声明
① 凡本网注明稿件来源为"原创"的所有文字、图片和音视频稿件,版权均属本网所有。任何媒体、网站或个人转载、链接转贴或以其他方式复制发表时必须注明"稿件来源:我考网",违者本网将依法追究责任;
② 本网部分稿件来源于网络,任何单位或个人认为我考网发布的内容可能涉嫌侵犯其合法权益,应该及时向我考网书面反馈,并提供身份证明、权属证明及详细侵权情况证明,我考网在收到上述法律文件后,将会尽快移除被控侵权内容。