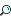Valence Bond Theory
Another topic that you’ll need to be familiar with for the SAT II Chemistry test is that of valence bond theory. By now, you are aware that two atoms will form a bond when there is orbital overlap between them, and a maximum of two electrons can be present in the overlapping orbitals. Since the pair of electrons is attracted to both atomic nuclei, a bond is formed, and as the extent of overlap increases, the strength of the bond increases. The electronic energy drops as the atoms approach each other, but it begins to increase again when they become too close. This means there is an optimum distance, the observed bond distance, at which the total energy is at a minimum. Let’s delve a little more deeply into sigma bonds now and describe them in more detail. As you know, sigma (s) bonds are single bonds. They result from the overlap of two s orbitals, an s and a p orbital, or two head-to-head p orbitals. The electron density of a sigma bond is greatest along the axis of the bond. Maximum overlap forms the strongest-possible sigma bond, and the two atoms will arrange themselves to give the greatest-possible orbital overlap. This is tricky with p orbitals since they are directional along the x, y, and z axes. Hybrid orbitals result from a blending of atomic orbitals (in other words, s and p orbitals) to create orbitals that have energy that’s in between the energy of the lone orbitals. Look at the methane molecule, for example: all four of the C—H bonds are 109.5o apart, while nonbonded p orbitals are only 90o apart.

The orbitals shown at the left of the figure are for a nonbonded carbon atom, but once the carbon atom begins to bond with other atoms (in this case hydrogen), the atomic orbitals hybridize, and this changes their shape considerably. Notice how the first set of figures form the sp3 atomic orbital, the hybrid, and this leads to further hybridization.

Ammonia also has sp3 hybridization, even though it has a lone pair.

Multiple Bonding Now let’s look more closely at pi bonds. As we mentioned earlier in this chapter, pi (p) bonds result from the sideways overlap of p orbitals, and pi orbitals are defined by the region above and below an imaginary line connecting the nuclei of the two atoms. Keep in mind that pi bonds never occur unless a sigma bond has formed first, and they may form only if unhybridized p orbitals remain on the bonded atoms. Also, they occur when sp or sp2 hybridization is present on central atom but not sp3 hybridization. Below, we show the formation of a set of sp2 orbitals. This molecule would contain a double bond, like ethene. Notice again how the first set of figures form the sp2 atomic orbital, the hybrid, and the last figure shows full hybridization:

The set of p orbitals that are unhybridized are not shown in this depiction:

A different view, which doesn’t show the hydrogens and centers on the C atoms, shows the unhybridized p orbitals that create the sideways overlap that’s necessary to create the double pi bond:

Here’s how it looks with all the pieces put together:

Here is a table summarizing hybridization and structure:effective pairshybridizationgeometry
2splinear

3sp2trigonal planar

4sp3tetrahedral


5dsp3trigonal bipyramidal


6d2sp3octahedral


(责任编辑:申月月)
视频学习
我考网版权与免责声明
① 凡本网注明稿件来源为"原创"的所有文字、图片和音视频稿件,版权均属本网所有。任何媒体、网站或个人转载、链接转贴或以其他方式复制发表时必须注明"稿件来源:我考网",违者本网将依法追究责任;
② 本网部分稿件来源于网络,任何单位或个人认为我考网发布的内容可能涉嫌侵犯其合法权益,应该及时向我考网书面反馈,并提供身份证明、权属证明及详细侵权情况证明,我考网在收到上述法律文件后,将会尽快移除被控侵权内容。