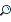Avogadro’s Law
The volume of a gas at a given temperature and pressure is directly proportional to the quantity of the gas. Avogadro’s hypothesis comes directly from this relationship, and you should definitely remember the following statement (which is an extrapolation of the above idea) for the SAT II Chemistry test: Equal volumes of gases under the same conditions of temperature and pressure contain equal numbers of molecules. Avogadro’s law, which is derived from this basic idea, says that the volume of a gas maintained at constant temperature and pressure is directly proportional to the number of moles of the gas, or
(责任编辑:申月月)
视频学习
我考网版权与免责声明
① 凡本网注明稿件来源为"原创"的所有文字、图片和音视频稿件,版权均属本网所有。任何媒体、网站或个人转载、链接转贴或以其他方式复制发表时必须注明"稿件来源:我考网",违者本网将依法追究责任;
② 本网部分稿件来源于网络,任何单位或个人认为我考网发布的内容可能涉嫌侵犯其合法权益,应该及时向我考网书面反馈,并提供身份证明、权属证明及详细侵权情况证明,我考网在收到上述法律文件后,将会尽快移除被控侵权内容。