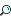Conjugate Acid-Base Pairs
Now that we’ve defined acids and bases, let’s discuss how they work together in reactions. Look at the generic acid-base reaction below:
HX(aq) + H2O(l) X-(aq) + H3O(aq)
When the forward reaction occurs, HX donates a proton to water (so it acts as the base) to form hydronium. When the reverse reaction occurs, the hydronium ion acts as the acid, donating a proton to the X-. Together, HX and X- are said to be conjugate acid-base pairs. Conjugate acid-base pairs are compounds that differ by the presence of one proton, or H+. All acids have a conjugate base, which is formed when their proton has been donated; likewise, all bases have a conjugate acid, formed after they have accepted a proton. Example Apply the appropriate acid-base theory to first identify the acid and base reacting and then identify the conjugate acid-base pairs in the examples below: ExplanationIn this first reaction, we see that HNO3 gives a proton to water, which then forms a hydronium ion. This makes HNO3 the acid in the forward reaction, and water acts as the base. HNO3’s conjugate base is , and water’s conjugate acid is the hydronium ion, or

Here donates the proton to water, so in the forward reaction it acts as the acid, and water is still the base. ’s conjugate base is NH3, and water’s conjugate acid is again the hydronium ion, H3O+.

Relative Strengths of Acids and Bases Certain acids are stronger than other acids, and some bases are stronger than others. What this means is that some acids are better at donating a proton, and some bases are better proton acceptors. A strong acid or base dissociates or ionizes completely in aqueous solution. A weak acid or base does not completely ionize. Strong Acids There are six strong acids that you’ll need to memorize for the SAT II Chemistry test:Hydrohalic acids: HCl, HBr, HI Nitric acid: HNO3Sulfuric acid: H2SO4Perchloric acid: HClO4 Let’s take a closer look at how acids differ in strength by focusing on perchloric acid. In general, the greater the number of oxygen atoms in a polyatomic ion, the stronger the acid.

So HClO4 is stronger than HClO3, which is stronger than HClO2, which is stronger than HClO. (Perchloric acid is the strongest among the six, but all the other oxyacids of chlorine are not considered strong acids.) Now think about why, as you take away oxygens, the strength of the acid decreases. The hydrogen (proton) to be removed is bonded to an oxygen atom. The oxygens are highly electronegative and are pulling the bonded pair of electrons away from the site where the hydrogen is bonded, thus making it easier to remove the H+. As the number of oxygen decreases, the molecule becomes less polar, and the H+ is harder to remove. Strong Bases There are fewer strong bases to memorize for the exam. These are hydroxides (—OH), oxides of 1A and 2A metals (except Mg and Be), H-, and . Remember that the stronger the acid, the weaker its conjugate base, and the converse is also true. The figure below illustrates the relative strengths of some common conjugate acid-base pairs.

The pH Scale As you know, water can act as either a proton donor (in the form of the hydronium ion, H3O+) or a proton acceptor (as OH-). In solution, a water molecule can even donate a proton to or accept a proton from another water molecule, and this process is called autoionization:
2H2OH3O+ + OH-
Since this reaction takes place in equilibrium, we can write an equilibrium expression, Keq, for it:
Keq = [H3O+][OH-]

And since this expression refers specifically to the ionization of water, we can write the equilibrium expression as Kw. At 25oC, the value of Kw, which is known as the ion-product constant, is 110-14. This means that the [H3O+] = [OH-] and each is equal to 110-7. When the concentrations of H+ and OH- are equal in a solution, the solution is said to be neutral. In acidic solutions, the concentration of H+ is higher than that of OH-, and in basic solutions, the concentration of OH- is greater than that of H+. The pH of a solution is calculated as the negative logarithm in base 10 of the hydronium ion concentration—it is an expression of the molar concentration of H+ ions in solution:
pH = -log [H+] or -log [H3O+]
A solution like the equilibrium expression for water, which is neutral at standard temperature, would have a pH of
pH = -log [110-7] = -(-7.00) = 7.00
So as you can see, neutral solutions have a pH of 7. If the solution contains more hydronium ions than this neutral solution ([H+] > 110-7), the pH will be less than 7.00, and the solution will be acidic; if the solution contains more hydroxide ions than this neutral solution ([OH-] > 110-7), the pH will be greater than 7.00, and the solution will be basic. Similarly, the pOH of a solution is calculated as the negative logarithm in base 10 of the hydroxide ion concentration:
pOH = -log [OH-]
and pH and pOH are related to each other by the equation
pH + pOH = 14
Since you won’t be allowed to have a calculator for the SAT II Chemistry test, you can use the following equation if you need to calculate the hydronium ion concentration of a solution:
[H3O+] = 10-pH
Now try a problem: What is the pH of a solution at 25oC in which [OH-] = 1.010-5 M? Explanation The fact that this solution is at 25oC tells us that we should use the Kw relationships. If the [OH-] = 1.010-5 M, then pOH = 5. You know that 1.010-5 is the same as plain old 10-5. The log of 10-5 is -5 (simply use the exponent when a number, any number, is written as 10power, so the “negative” of the log is equal to -(-5), or simply 5. Now, if the pOH is 5, then the pH is 9 since pH + pOH = 14.
(责任编辑:申月月)
视频学习
我考网版权与免责声明
① 凡本网注明稿件来源为"原创"的所有文字、图片和音视频稿件,版权均属本网所有。任何媒体、网站或个人转载、链接转贴或以其他方式复制发表时必须注明"稿件来源:我考网",违者本网将依法追究责任;
② 本网部分稿件来源于网络,任何单位或个人认为我考网发布的内容可能涉嫌侵犯其合法权益,应该及时向我考网书面反馈,并提供身份证明、权属证明及详细侵权情况证明,我考网在收到上述法律文件后,将会尽快移除被控侵权内容。